Grades:
11th Grade, 12th Grade
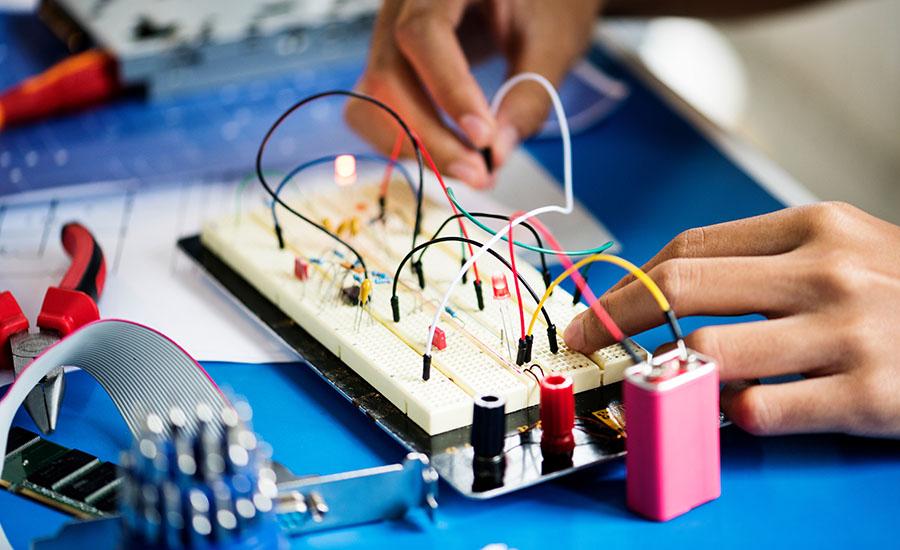
In this high school chemistry lesson, students will explore and compare different types of batteries, including lithium-ion, zinc-air/copper-zinc, and sustainable alternatives. Through guided research


