
Chemiluminescence
In a chemiluminescence chemical reaction, light is generated as a product. What causes light to be generated from chemicals that interact? This chemistry lesson explores the reasons why light is generated through a review of electron movement, ion formation, photon absorption and emission, with an introduction to oxidation-reduction reactions that characterize chemiluminescence. The lesson includes a demonstration of chemiluminescence reactions. In addition to the one day lesson, there is a one day lab component in which students explore the relationship between glow stick reaction rates with temperature and external light.
Lesson Grade Level
11th GradeLesson Plan Link/URL
https://docs.google.com/presentation/d/1M72zjMfK4rtyo-giLdaNwq7dS1KpdLMI/edit?u…Related Content
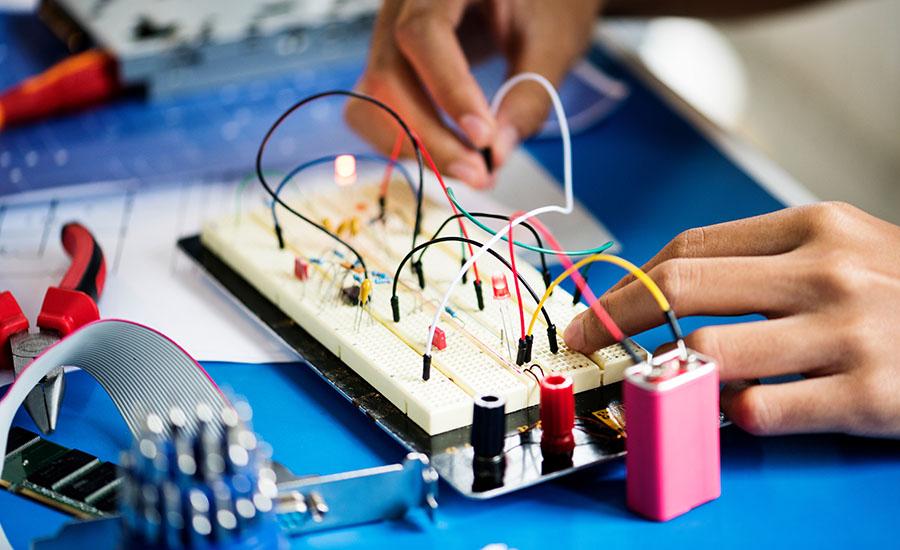
This hands-on lesson helps students understand exponential functions by using an LED circuit. LED luminosity decreases at an exponential rate as more are added in series. As students add more lights

It can be challenging to sort through the various robot options out in the market for educators. This lesson is for educators who have used robots or are new to using robots. The intention is to use

Students will explore mathematical rules for combining fractions with unlike denominators by modeling equivalent resistance calculations for resistors wired in parallel electrical circuits. Using a