
Chemiluminescence
by Donna Brunjes
In a chemiluminescence chemical reaction, light is generated as a product. What causes light to be generated from chemicals that interact? This chemistry lesson explores the reasons why light is generated through a review of electron movement, ion formation, photon absorption and emission, with an introduction to oxidation-reduction reactions that characterize chemiluminescence. The lesson includes a demonstration of chemiluminescence reactions. In addition to the one day lesson, there is a one day lab component in which students explore the relationship between glow stick reaction rates with temperature and external light.
Lesson Grade Level
11th GradeLesson Plan Link/URL
https://docs.google.com/presentation/d/1M72zjMfK4rtyo-giLdaNwq7dS1KpdLMI/edit?u…
Featured
Off
Related Content

Grades:
9th Grade, 10th Grade, 11th Grade, 12th Grade
In this lesson students evaluate the advantages and disadvantages of conventional, petroleum-based plastics, bioplastics, and their different varieties. The lesson is driven by class/group research
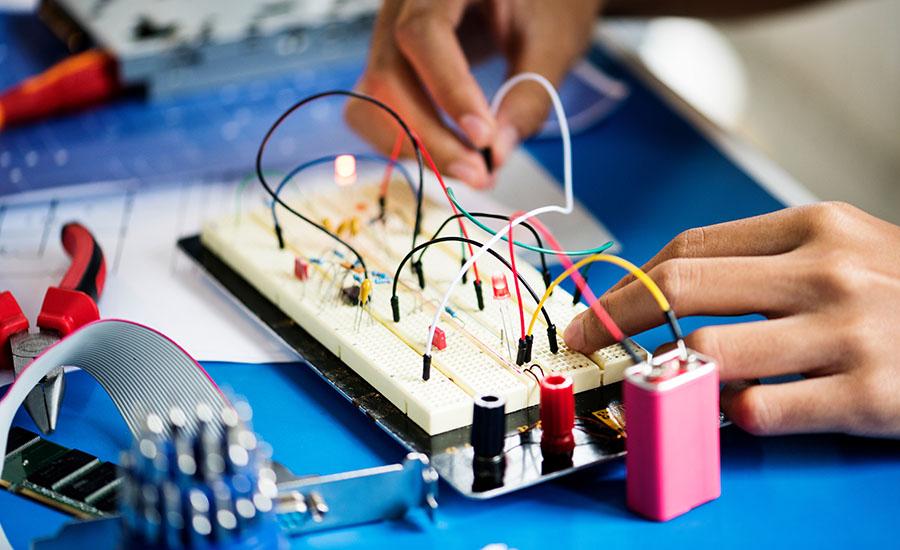
Grades:
7th Grade, 8th Grade, 9th Grade, 10th Grade, 11th Grade, 12th Grade
The purpose of this project is to have students use their knowledge of series and parallel circuits to create an electronic greeting card or an electronic game. This lesson should be given after

Grades:
10th Grade, 11th Grade, 12th Grade
Students will unravel the intricacies of environmental impact analysis, gaining insight into its pivotal role in evaluating the sustainability of chemical processes. Through a blend of explanation