
Chemiluminescence
In a chemiluminescence chemical reaction, light is generated as a product. What causes light to be generated from chemicals that interact? This chemistry lesson explores the reasons why light is generated through a review of electron movement, ion formation, photon absorption and emission, with an introduction to oxidation-reduction reactions that characterize chemiluminescence. The lesson includes a demonstration of chemiluminescence reactions. In addition to the one day lesson, there is a one day lab component in which students explore the relationship between glow stick reaction rates with temperature and external light.
Lesson Grade Level
11th GradeLesson Plan Link/URL
https://docs.google.com/presentation/d/1M72zjMfK4rtyo-giLdaNwq7dS1KpdLMI/edit?u…Related Content

This lesson plan will equip the students with the basic understanding of the forms of electromagnetic waves. It also includes a hands-on activity on assessing EMF radiation levels in classrooms that
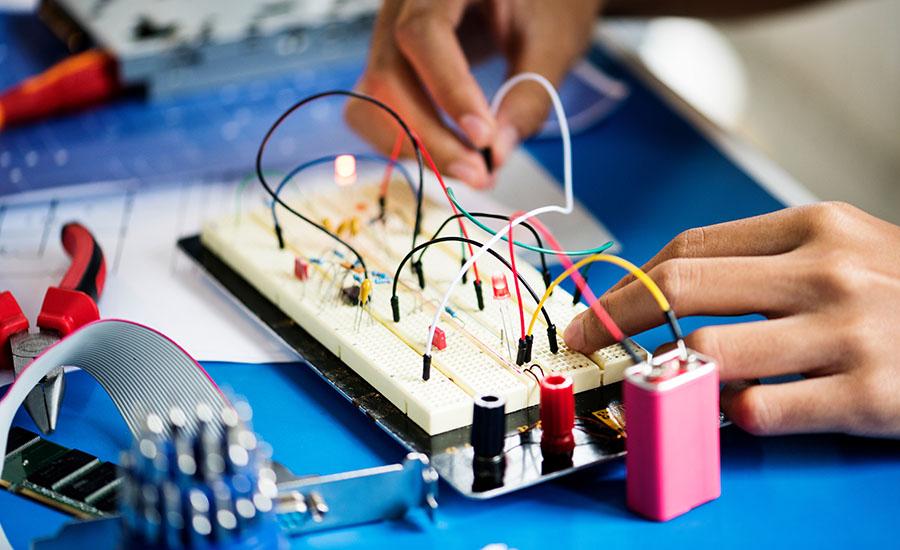
In this lesson, students will explore how energy works using circuits and stored energy.

This multi-day activity is designed for an introductory 4th grade STEM after school club. It can be easily modified for whole classes and for lower and upper grade levels. The activity has students